THINPREP PAP TEST
With loyalty to our corporate philosophy regarding the inquiring and promotion of innovative products that represent the future of medical diagnosis, and by developing, since1998, a steady partnership with Hologic Inc., a top firm in developing diagnostic and therapeutic technologies in Women’s Health worldwide, we distribute in Greece ThinPrep.Pap.Test, the innovative diagnostic test for cervical cancer.
ThinPrep is and accurate and technologically advanced method of prevention and timely diagnosis for cervical cancer.
It is the evolution of the typical Pap Test. It is more accurate than the typical Pap test because it can identify more damages or lesions related to cervical cancer or precancerous lesions 1.
ThinPrep Pap Test was approved by the USA Food and Drug Administration (FDA) in 1996 and it was the first Liquid Based Cytology Test.
Since it was introduced in 1996, ThinPrep Pap Test contributed in reducing cervical cancer up to 30% 1.
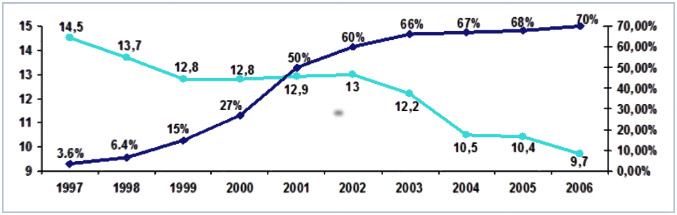
Source: 1 Surveillance, Epidemiology, and End Results (SEER) Program. SEER Database: Incidence – SEER 9 Regs Public-Use, Nov. 2004 Sub (1973-2002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2005, based on November 2004 submission.
― An exclusive method of gynecological testing in the biggest hospitals and diagnostic centers in Greece.
Since 2015, in cooperation with Generali Hellas, Thin Prep has become the exclusive method for conducting gynaecological testing, both for prevention and for diagnosis in the largest hospitals and diagnostic centers in Greece

For more information about ThinPrep


